Which Best Describes the Acid-base Classification of Ammonia
Ammonia is a base NH3. Reaction is given below Reaction is given below Reaction of Boron trifluoride Lewis acid with fluoride ion Lewis base Octet of Boron in Boron trifluoride BF 3 is incomplete so it acts as a very good Lewis acid and combines with fluoride ion and.

Solved Which Of The Following Reactions Best Describes The Chegg Com
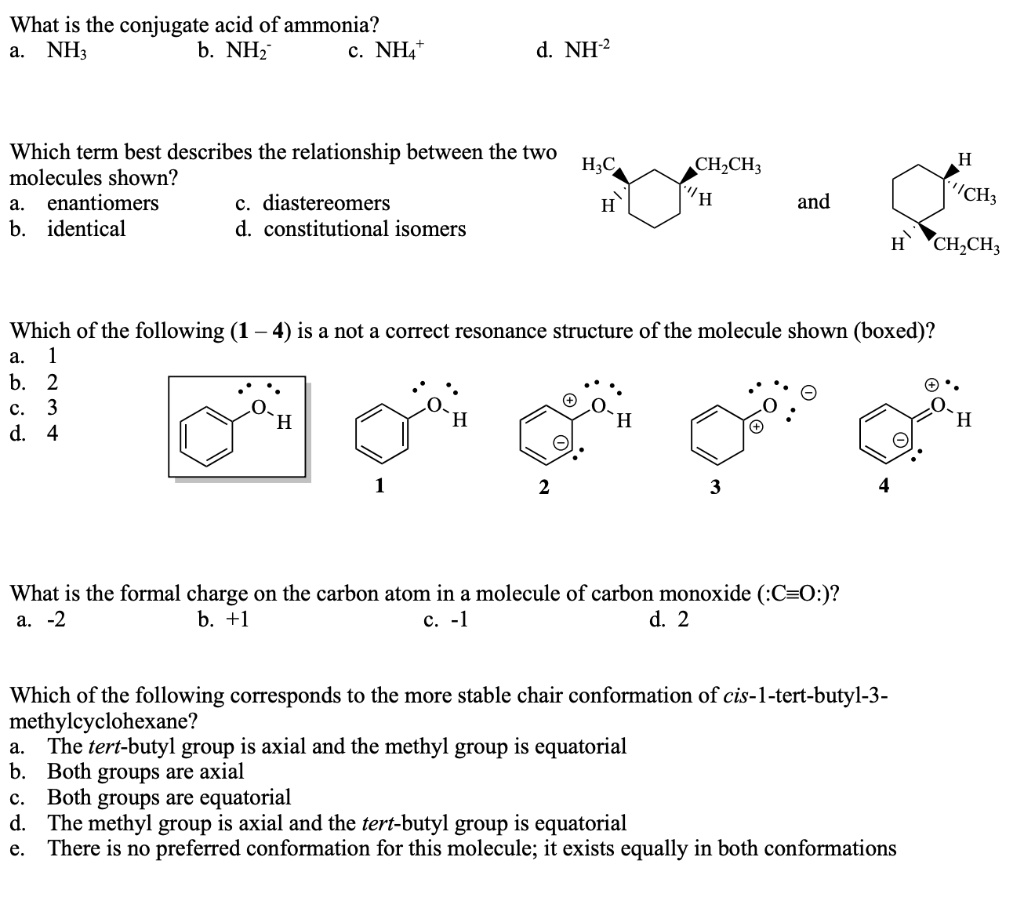
The ammonium ion is the conjugate acid this hydrogen ion should be produced again in order to reform the ammonia.

. Looking at it from the other side the ammonium ion is an acid and ammonia is its conjugate base. Muriatic acid and ammonia. For this reason ammonia is considered basic because its nitrogen atom has an electron pair that readily accepts a proton.
What class of compounds is primarily responsible for the pleasant smell of many fruits. Amino Acids Definition Structure Properties Classification. Due to the NH3 molecules polarity and its ability to form hydrogen bonds ammonia is strongly soluble in water.
Which of the following reactions best describes the acid-base properties of ammonia NH_4 in aqueous solution. Temperature and salinity also affect the proportion of NH 4. Theories of acid-base indicators as Weak acids and weak bases such as acetic acid and ammonia do not dissociate completely in water.
Ammonia is a foundation since the water accepts hydrogen ions. Ammonia is moderately basic. Acid-base homeostasis is critical for normal physiology and health.
An acid is a substance which ionises or dissociates in water to produce hydrogen ions H. Both the reaction of an acid chloride with an amine and the reaction of an acid anhydride with ammonia are correct. Which of the following compositions of cleaners is the best choice if you have hard water.
The strength of an acid or base depends on _____. It is a proton donor and forms a conjugate base Ammonia NH3 in most reactions. B When an acid reacts with a base neutralisation takes place.
A When a base reacts with an ammonium salt ammonia is given off. Hence multiple often redundant pathways and processes exist to control systemic pH. Ammonia is a BasicSo option B is your answerHope this helps.
Ammonia can be used as a fuel and fertilizer. NH_3aq H_2Ol NH_4aq OH-aq NH_3aq 3H_2Ol N3-aq 3H_2Oaq NH_3aq 3H_2Ol N aq 3H_2O aq NH_3aq rightarrow N aq 3H aq What is the net ionic equations for the complete neutralization of. Chemistry questions and answers.
Ammonia is a compound with the formula NH3. The hydroxide ion can accept a hydrogen ion to reform the water. The hydroxide ion is a base and water is its conjugate acid.
Ammonia is norally encountered as a. Strong acids produce a lot of hydrogen ions in solution while strong bases produce a lot of hydroxide ions in solution and a small number of hydrogen ions. Muriatic acid is industrial hydrochloric acid HCl.
The water is acting as an acid and its conjugate base is the hydroxide ion. A biomolecule sometimes known as a biological molecule is a term that refers to molecules found in living things that are required for one or more biological processes such as cell division morphogenesis or development. View acides bases and salts QS 1pdf from PS 147 at Institute of Education Main Campus Khairpur.
Can ammonia act as an acid. A 10 M aqueous solution has a pH of 116 and if a strong acid is added to such a solution until the solution is neutral pH 7 994 of the ammonia molecules are protonated. Similarly Cl like ammonia can accept a proton and hence a base.
However ammonia is classified as a weak base which is a chemical compound that doesnt completely break apart into ions in an aqueous solution. C When an acid reacts with a carbonate carbon dioxide is given off. D When the acidity of a solution increases the pH increases.
Since it has come from the base ammonia it is called as a conjugate acid of base ammonia. It is considered as the conjugate base of the acid HCl. A long hydrocarbon chain with sodium salt B long hydrocarbon chain with potassium salt.
It fits all 3 acid-base theories even though it would still be acidic if it satisfied only Lewis. They are referred to as weak acids or weak bases. _ acids bases and salts CHEMISTRY OXFORD IGCSE HEBA.
Eddie should insert baking soda pH. The Swedish chemist Arrhenius proposed the following definition of an acid. Ammonia is a compound that is normally encountered as a gas with a characteristic pungent odor.
Eddie must decide where add baking soda pH 9. The lone pair makes ammonia a base a proton acceptor. Derangements in acid-base homeostasis however are common in clinical medicine and can often be related to the systems involved in acid-base transport in the kidneys.
In water ammonium produces hydrogen ions. Nitrogen and hydrochloric acid. 12022016 Chemistry High School answered Ammonia is _____.
NH 4 like HCl can donate a proton and hence an acid. Ammonia is normally a base but in some reactions it can act like an. Water behaves as an acid and the hydroxide ion is the conjugate base.
Ammonia has a low boiling temperature at -33 degrees celsius and is lighter than air. What is the definition of an acid and a base. For example hydrochloric acid HClaq is a solution of hydrogen chloride in water obtained by dissolving pure hydrogen.
Which of the following reactions best describes the acid-base properties of ammonia NH3 in aqueous solution. NH3 aq 3H20 I N aq 3H30 aq NH3 aq H2O l - NH4 aq OH aq NH3 aq N3- aq 3H aq NH3 aq H2O l NH4 aq OH aq NH3 aq 3H2O 1 N3- aq 3H30 aq Question. In this article youll get to know about the acidity and basicity of NH3 in.
Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety. A base is any molecule that accepts a proton while an acid is any molecule that releases a proton. Although helpful to the nutritional needs of the Earth ammonia can cause serious health damage.
Ammonia D vitamin C. Often students wonder about whether NH3 is an acid or base. The reaction of H Lewis acid and NH 3 Lewis base Ammonia combines with hydrogen ions and forms ammonium ions.
NH3 also known as Ammonia is a pungent-smelling gas compound which comprises of 1 atom of Nitrogen and 3 atoms of Hydrogen. A crude nonacidic product mixture dissolved in chloroform contains acetic acid. A how many hydrogen atoms are contained in each molecule of the acid or base.
Is muriatic acid same as ammonia. Large macromolecules or polyanions like proteins. Eddies chemistry teacher has asked the class to add various household substances to this scale of acids and bases.
Which best describes the chemical behavior of amines. An acid a base a neutral 2 See answers Advertisement Advertisement SJ2006 SJ2006 Ammonia is a Basic So option B is your answer. The Bronsted Lowry transfer can be written as-.

2 Polar Covalent Bonds Acids And Bases Ppt Download
Solved Tools None U O Na 0 H2 O Nh3 O Make Ammonia Chegg Com
Solved What Is The Conjugate Acid Of Ammonia Nh Nhz Nh4 Which Term Best Describes The Relationship Between The Two H C Molecules Shown Enantiomers Diastereomers B Identical Constitutional Isomers Ch Ch H And
No comments for "Which Best Describes the Acid-base Classification of Ammonia"
Post a Comment